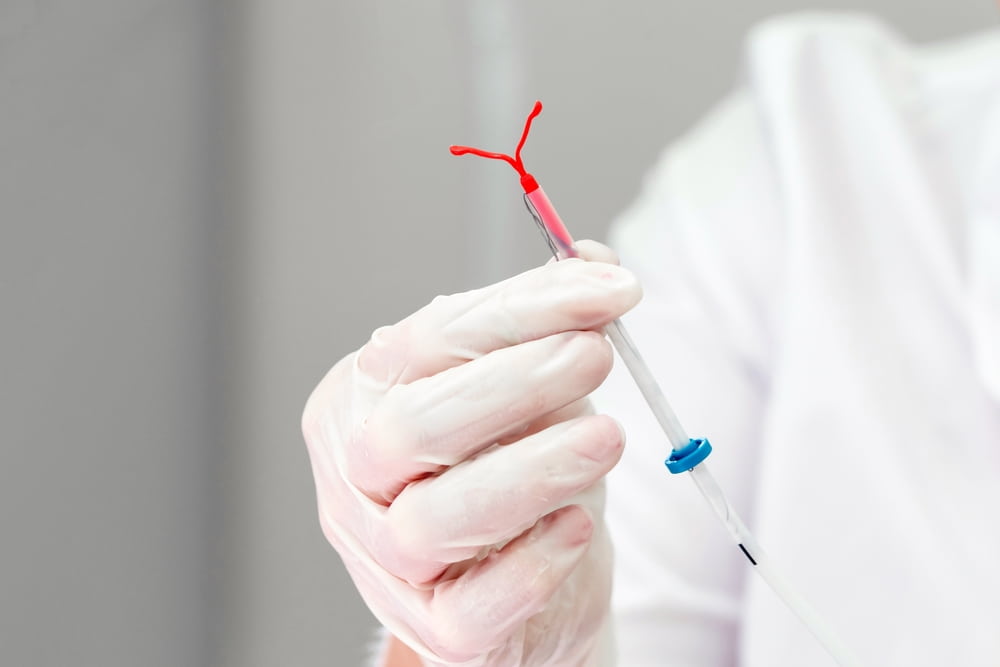
A federal jury in Atlanta found in favor of Teva Pharmaceuticals USA Inc. in the first bellwether trial over claims the company’s Paragard intrauterine contraceptive device was prone to breaking apart inside people’s bodies during the removal process.
The trial in the US District Court for the Northern District of Georgia was the first of three bellwether cases meant to test the claims brought in the MDL involving more than 3,800 lawsuits. Plaintiffs allege Teva failed to warn women about the potential breakage of the IUD, causing injuries that can impact fertility and often require surgical removal.
The jury rejected plaintiff Pauline Rickard’s attempt to hold the company liable for allegedly failing to warn her about problems with its product. Rickard sued Teva in 2021, claiming she needed multiple procedures to remove her Paragard IUD.
Teva has argued that the plaintiffs’ claims are preempted by federal law governing drug labeling and said the company adequately warned physicians of the risk of breakage.
Plaintiffs will have more opportunities to prove their claims at the next bellwether trials. An attorney for Rickard, Erin Copeland, said the verdict will not stop the legal battle against Teva.
“We will continue to expose what has been buried in the company documents, including the conduct that kept women in the dark, and demand accountability, regardless of the outcome of any one trial,” Copeland said.